Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Which of the following is NOT a physical property of water?
a. | It has a boiling point of 100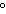 C. C. | b. | It is a colorless
liquid. | c. | It is composed of hydrogen and oxygen. | d. | Sugar dissolves in
it. |
|
|
|
2.
|
Which state of matter takes both the shape and volume of its container?
a. | solid | c. | gas | b. | liquid | d. | both b and c |
|
|
|
3.
|
Which of the following is used for chemical symbols today?
a. | drawings | c. | letters | b. | icons | d. | numbers |
|
|
|
4.
|
Which of the following represents a compound?
|
|
|
5.
|
Which of the following is a chemical property?
a. | color | c. | freezing point | b. | hardness | d. | ability to react with
oxygen |
|
|
|
6.
|
A chemical change occurs when a piece of wood ____.
a. | is split | c. | decays | b. | is painted | d. | is cut |
|
|
|
7.
|
Which of the following does NOT indicate that a chemical change may have taken
place?
a. | fracture formation | c. | precipitate formation | b. | gas
production | d. | energy
transfer |
|
|
|
8.
|
Which group of measurements is the most precise? (Each group of measurements is
for a different object.)
a. | 2 g, 3 g, 4 g | c. | 2 g, 2.5 g, 3 g | b. | 2.0 g, 3.0 g, 4.0 g | d. | 1 g, 3 g, 5 g |
|
|
|
9.
|
What is the volume of a salt crystal measuring 2.44 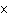 10 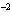 m by
1.4 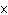 10 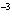 m by 8.4 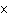 10 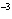 m?
|
|
|
10.
|
A vapor is which state of matter?
a. | solid | c. | gas | b. | liquid | d. | all of the
above |
|
|
|
11.
|
A substance that forms a vapor is generally in what physical state at room
temperature?
a. | solid | c. | gas | b. | liquid | d. | liquid or solid |
|
|
|
12.
|
Which of the following is a heterogeneous mixture?
a. | air | c. | steel | b. | salt water | d. | soil |
|
|
|
13.
|
What is the result of multiplying 2.5 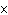 10 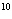 by 3.5 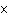
10 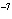 ?
|
|
|
14.
|
Which of the following measurements is expressed to three significant
figures?
a. | 0.007 m | c. | 7.30 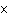 10 10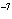 km km | b. | 7077
mg | d. | 0.070
mm |
|
|
|
15.
|
What is the measurement 111.009 mm rounded off to four significant
digits?
a. | 111 mm | c. | 111.01 mm | b. | 111.0 mm | d. | 110 mm |
|
|
|
16.
|
Express the product of 4.0 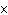 10 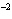 m and 8.1 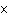
10  m using the correct number of significant digits.
|
|
|
17.
|
Which of the following volumes is the smallest?
a. | one microliter | c. | one milliliter | b. | one liter | d. | one deciliter |
|
|
|
18.
|
An example of an extensive property of matter is ____.
a. | temperature | c. | mass | b. | pressure | d. | hardness |
|
|
|
19.
|
Which state of matter has a definite volume and takes the shape of its
container?
a. | solid | c. | gas | b. | liquid | d. | both b and c |
|
|
|
20.
|
Which of the following is true about homogeneous mixtures?
a. | They are known as solutions. | b. | They consist of two or more
phases. | c. | They have compositions that never vary. | d. | They are always
liquids. |
|
|
|
21.
|
Which of the following measurements contains two significant figures?
a. | 0.004 00 L | c. | 0.000 44 L | b. | 0.004 04 L | d. | 0.004 40 L |
|
|
|
22.
|
What quantity is represented by the metric system prefix deci-?
|
|
|
23.
|
The weight of an object ____.
a. | is the same as its mass | c. | is not affected by
gravity | b. | depends on its location | d. | is always the same |
|
|
|
24.
|
What is the volume of 80.0 g of ether if the density of ether is 0.70
g/mL?
|
|
|
25.
|
If the temperature of a piece of steel decreases, what happens to its
density?
a. | The density decreases. | b. | The density increases. | c. | The density does not
change. | d. | The density first increases, then decreases. |
|