Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Which of the following is a property of an acid?
a. | sour taste | c. | strong color | b. | nonelectrolyte | d. | unreactive |
|
|
|
2.
|
What is an acid according to Arrhenius?
a. | a substance that ionizes to yield protons in aqueous solution | b. | a substance that is
a hydrogen ion donor | c. | a substance that accepts an electron
pair | d. | a substance that is a hydrogen ion acceptor |
|
|
|
3.
|
Which of these is an Arrhenius base?
|
|
|
4.
|
What type of acid is sulfuric acid?
a. | monoprotic | c. | triprotic | b. | diprotic | d. | none of the
above |
|
|
|
5.
|
Which compound can act as both a Brønsted-Lowry acid and a
Brønsted-Lowry base?
a. | water | c. | sodium hydroxide | b. | ammonia | d. | hydrochloric
acid |
|
|
|
6.
|
Which type of solution is one with a pH of 8?
a. | acidic | b. | basic | c. | neutral | d. | The type varies, depending on the
solution. |
|
|
|
7.
|
Which of these solutions is the most basic?
|
|
|
8.
|
What characterizes a strong acid or base?
a. | polar covalent bonding | b. | complete ionization in
water | c. | ionic bonding | d. | presence of a hydroxide or hydrogen
ion |
|
|
|
9.
|
Acetic acid ionizes in water as follows: 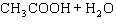  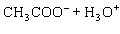 Fewer than 1% of ethanoic acid molecules are ionized at any instant. The acetate ion
(CH 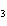 COO 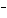 ) is therefore ____. a. | a poor hydrogen-ion acceptor | c. | a poor hydrogen-ion
donor | b. | a good hydrogen-ion acceptor | d. | a good hydrogen-ion
donor |
|
|
|
10.
|
Which of the following pairs consists of a weak acid and a strong base?
a. | sulfuric acid, sodium hydroxide | c. | acetic acid, sodium
hydroxide | b. | acetic acid, ammonia | d. | nitric acid, calcium hydroxide |
|
|
|
11.
|
A substance with a K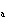 of 1 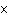 10 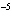 would be
classified as a ____. a. | strong acid | c. | strong base | b. | weak acid | d. | weak base |
|
|
|
12.
|
If an acid has a K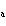 = 1.6 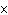 10 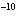 , what is
the acidity of the solution? a. | acidic | c. | neutral | b. | basic | d. | The answer cannot be
determined. |
|
|
|
13.
|
Which base is strong, but never concentrated?
a. | magnesium hydroxide | c. | ammonia | b. | sodium hydroxide | d. | water |
|
|
|
14.
|
The process of adding a known amount of solution of known concentration to
determine the concentration of another solution is called ____.
a. | neutralization | c. | titration | b. | hydrolysis | d. | buffer capacity |
|
|
|
15.
|
What kind of ion is contained in salts that produce an acidic solution?
a. | a positive ion that releases a proton to water | b. | a negative ion that
releases a proton to water | c. | a positive ion that attracts a proton from
water | d. | a negative ion that attracts a proton from water |
|
Numeric Response
|
|
|
16.
|
What is the pH when the hydrogen ion concentration is 7.0 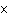 10 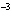 M M?
|