Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
What is the name given to the electrons in the highest occupied energy level of
an atom?
a. | orbital electrons | c. | anions | b. | valence electrons | d. | cations |
|
|
|
2.
|
What is the electron configuration of the calcium ion?
|
|
|
3.
|
The octet rule states that, in chemical compounds, atoms tend to have
____.
a. | the electron configuration of a noble gas | b. | more protons than
electrons | c. | eight electrons in their principal energy level | d. | more electrons than
protons |
|
|
|
4.
|
What is the formula of the ion formed when potassium achieves noble-gas electron
configuration?
|
|
|
5.
|
What is the formula of the ion formed when tin achieves a stable electron
configuration?
|
|
|
6.
|
How many electrons does nitrogen gain in order to achieve a noble-gas electron
configuration?
|
|
|
7.
|
The electron configuration of a fluoride ion, F 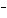 , is
____.
|
|
|
8.
|
A compound held together by ionic bonds is called a ____.
a. | diatomic molecule | c. | covalent molecule | b. | polar compound | d. | salt |
|
|
|
9.
|
Which of the following is true about the melting temperature of potassium
chloride?
a. | The melting temperature is relatively high. | b. | The melting
temperature is variable and unpredictable. | c. | The melting temperature is relatively
low. | d. | Potassium chloride does not melt. |
|
|
|
10.
|
Which of the following elements can form diatomic molecules held together by
triple covalent bonds?
a. | carbon | c. | fluorine | b. | oxygen | d. | nitrogen |
|
|
|
11.
|
Which elements can form diatomic molecules joined by a single covalent
bond?
a. | hydrogen only | b. | halogens only | c. | halogens and members
of the oxygen group only | d. | hydrogen and the halogens
only |
|
|
|
12.
|
When one atom contributes both bonding electrons in a single covalent bond, the
bond is called a(n) ____.
a. | one-sided covalent bond | c. | coordinate covalent
bond | b. | unequal covalent bond | d. | ionic covalent bond |
|
|
|
13.
|
The side-by-side overlap of p orbitals produces what kind of bond?
a. | alpha bond | c. | pi bond | b. | beta bond | d. | sigma bond |
|
|
|
14.
|
Where are the electrons most probably located in a molecular bonding
orbital?
a. | anywhere in the orbital | b. | between the two atomic
nuclei | c. | in stationary positions between the two atomic nuclei | d. | in circular orbits
around each nucleus |
|
|
|
15.
|
Sigma bonds are formed as a result of the overlapping of which type(s) of atomic
orbital(s)?
a. | s only | c. | d only | b. | p only | d. | s and
p |
|
|
|
16.
|
The shape of the methane molecule is called ____.
a. | tetrahedral | c. | four-cornered | b. | square | d. | planar |
|
|
|
17.
|
What causes water molecules to have a bent shape, according to VSEPR
theory?
a. | repulsive forces between unshared pairs of electrons | b. | interaction between
the fixed orbitals of the unshared pairs of oxygen | c. | ionic attraction and
repulsion | d. | the unusual location of the free electrons |
|
|
|
18.
|
What is the shape of a molecule with a triple bond?
a. | tetrahedral | c. | bent | b. | pyramidal | d. | linear |
|
|
|
19.
|
What type of hybridization occurs in the orbitals of a carbon atom participating
in a triple bond with another carbon atom?
|
|
|
20.
|
How many pi bonds are formed when sp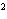
hybridization occurs in ethene, C 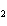 H 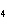 ?
|
|
|
21.
|
A bond formed between a silicon atom and an oxygen atom is likely to be
____.
a. | ionic | c. | polar covalent | b. | coordinate covalent | d. | nonpolar
covalent |
|
|
|
22.
|
Which of the following covalent bonds is the most polar?
|
|
|
23.
|
What are the weakest attractions between molecules?
a. | ionic forces | c. | covalent forces | b. | Van der Waals forces | d. | hydrogen forces |
|
|
|
24.
|
What causes hydrogen bonding?
a. | attraction between ions | b. | motion of electrons | c. | sharing of electron
pairs | d. | bonding of a covalently bonded hydrogen atom with an unshared electron
pair |
|
|
|
25.
|
What is required in order to melt a network solid?
a. | breaking Van der Waals bonds | c. | breaking hydrogen
bonds | b. | breaking ionic bonds | d. | breaking covalent bonds |
|
Essay
|
|
|
26.
|
Explain how a pure metal is held together. Include a definition of a metallic
bond in your explanation.
|
|
|
27.
|
Can some atoms exceed the limits of the octet rule in bonding? If so, give an
example.
|
|
|
28.
|
Explain what is meant by VSEPR theory. Give an example of how VSEPR theory can
be applied to predict the shape of a molecule.
|