Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
A ketone has the general structure ____________.
|
|
|
2.
|
The names of the straight-chain alkanes all end with the suffix ____.
|
|
|
3.
|
When an equation is used to calculate the amount of product that will form
during a reaction, then the value obtained is called the ____.
a. | minimum yield | c. | actual yield | b. | percent yield | d. | theoretical
yield |
|
|
|
4.
|
Which of the following compounds is trichloromethane?
|
|
|
5.
|
Which of the following will evaporate the fastest?
a. | water at 20 C C | b. | water at 40 C C | c. | water at 0 C C | d. | All samples will evaporate at the same
rate. |
|
|
|
6.
|
In every balanced chemical equation, each side of the equation has the same
number of ____.
a. | moles | c. | atoms of each element | b. | coefficients | d. | molecules |
|
|
|
7.
|
Which carbon skeleton contains a carboxyl group?
|
|
|
8.
|
What is shown by the structural formula of a molecule or polyatomic ion?
a. | the number of metallic bonds | c. | the number of ionic
bonds | b. | the shapes of molecular orbitals | d. | the arrangement of bonded
atoms |
|
|
|
9.
|
Standard conditions when working with gases are defined as ____.
a. | 0 K and 1 kPa | c. | 0 C and 1 kPa C and 1 kPa | b. | 0 C and
101.3 kPa C and
101.3 kPa | d. | 0 K and 101.3
kPa |
|
|
|
10.
|
Pseudo-science rarely makes any ______.
a. | chemical reactions | c. | weird things happen | b. | cures for cancer | d. | progress |
|
|
|
11.
|
What is one of the most distinguishing features of science?
a. | biology | c. | Scientist always wear lab coats | b. | It rarely
changes | d. | It is
falsifiable |
|
|
|
12.
|
A molecule with a single covalent bond is ____.
|
|
|
13.
|
Which of the following best describes the claims of the faith healer from the
Secrets of the Psychics?
a. | They were absolutely true, he was healing people. | b. | They were absolutely
false, his wife was telling him all of his information. | c. | They were misguided,
he could only heal cancer, not arthritis. | d. | He yelled alot so they must be somewhat
true. |
|
|
|
14.
|
James Randi used to be a _____?
a. | Beard model | c. | Olympic synchronized swimmer | b. | Magician | d. | University professor |
|
|
|
15.
|
Compared to the melting points of ionic compounds, the melting points of
molecular solids tend to be ____.
a. | lower | c. | similar | b. | unpredictable | d. | higher |
|
|
|
16.
|
When the vapor pressure of a liquid is equal to the atmospheric pressure, the
liquid ____.
a. | has no observable changes. | c. | boils
vigorously. | b. | evaporates. | d. | begins to boil. |
|
|
|
17.
|
The volume of a gas is doubled while the temperature is held constant. How does
the gas pressure change?
a. | It is doubled. | b. | It does not change. | c. | It varies depending
on the type of gas. | d. | It is reduced by one
half. |
|
|
|
18.
|
How do atoms achieve noble-gas electron configurations in single covalent
bonds?
a. | Two atoms share one electron. | b. | Two atoms share two pairs of
electrons. | c. | One atom completely loses two electrons to the other atom in the
bond. | d. | Two atoms share two electrons. |
|
|
|
19.
|
The shape of the methane molecule is called ____.
a. | planar | c. | tetrahedral | b. | four-cornered | d. | square |
|
|
|
20.
|
Which of the following elements can form diatomic molecules held together by
triple covalent bonds?
a. | nitrogen | c. | fluorine | b. | oxygen | d. | carbon |
|
|
|
21.
|
How does the atmospheric pressure at altitudes below sea level compare with
atmospheric pressure at sea level?
a. | The atmospheric pressure below sea level is higher. | b. | The pressures are
the same. | c. | Differences in pressures cannot be determined. | d. | The atmospheric
pressure below sea level is lower. |
|
|
|
22.
|
Why do atoms share electrons in covalent bonds?
a. | to become ions and attract each other | b. | to become more polar | c. | to attain a
noble-gas electron configuration | d. | to increase their atomic
numbers |
|
|
|
23.
|
Scientists always get their funding from unbiased sources
a. | False | b. | Scientist are always self
funded | c. | All the above | d. | True |
|
|
|
24.
|
Chemical reactions ____.
a. | occur only in living organisms | c. | produce new
substances | b. | create and destroy atoms | d. | only occur outside living organisms |
|
|
|
25.
|
The temperature at which the motion of particles theoretically ceases is
____.
a. | 273 C C | c. | –273 K | b. | 0 C C | d. | 0
K |
|
|
|
26.
|
Sigma bonds are formed as a result of the overlapping of which type(s) of atomic
orbital(s)?
a. | s only | c. | d only | b. | p only | d. | s and
p |
|
|
|
27.
|
Which of the following is NOT an important fossil fuel?
a. | petroleum | c. | natural gas | b. | coal | d. | hydrogen |
|
|
|
28.
|
Science includes alot of ______?
a. | The occult | c. | Alien DNA | b. | Non-duplicatable
experiments | d. | Research and
testing |
|
|
|
29.
|
What compound is the simplest aromatic compound?
a. | methane | c. | ethene | b. | benzene | d. | ethyne |
|
|
|
30.
|
Which of these compounds is an alkene?
a. | nonene | c. | propanone | b. | methane | d. | butyne |
|
|
|
31.
|
Alkanes are hydrocarbons that contain what type of bonds?
a. | at least one double bond | c. | single covalent bonds
only | b. | ionic bonds | d. | at least one triple bond |
|
|
|
32.
|
A gas occupies a volume of 2.4 L at 14.1 kPa. What volume will the gas occupy at
84.6 kPa?
a. | 14 L | c. | 497 L | b. | 2.5 L | d. | 0.40 L |
|
|
|
33.
|
The reaction 2Fe 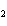 3Cl 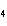 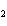 2FeCl  is an example of which type of
reaction? a. | combination reaction | c. | single-replacement reaction | b. | decomposition
reaction | d. | combustion
reaction |
|
|
|
34.
|
What is the general formula for a straight-chain alkane?
|
|
|
35.
|
When should scientific claims be taken personally
a. | Never | b. | If they have been insulted | c. | When you really know
that they are right | d. | When they are
challenged |
|
|
|
36.
|
The general name for hydrocarbons with at least one triple covalent bond is
____.
a. | alkanes | c. | alkyls | b. | alkynes | d. | alkenes |
|
|
|
37.
|
Which hydrocarbon rings are most common in nature?
a. | rings with 6 or 7 carbon atoms | c. | rings with 3 or 4 carbon
atoms | b. | rings with 5 or 6 carbon atoms | d. | rings with 4 or 5 carbon
atoms |
|
|
|
38.
|
Which carbon skeleton represents an ether?
a. |
 | c. |
 | b. |
CCCOCCC | d. |
none of the
above |
|
|
|
39.
|
A bond that is not symmetrical along the axis between two atomic nuclei is a(n)
____.
a. | beta bond | c. | alpha bond | b. | pi bond | d. | sigma bond |
|
|
|
40.
|
Companies still sell products and provide services that are bad for
people.
a. | Not true, the FDA doesn’t allow this. | b. | Large corporations
are evil, but not because they sell damaging products. | c. | True, the tobacco and tanning salon industries
are examples. | d. | Not true, cosmetics companies would never put female sex hormones in facial
creams! |
|
|
|
41.
|
Esters contribute which property to fruits?
a. | skin thickness | c. | texture | b. | odor | d. | color |
|
|
|
42.
|
Which of the following is a condensed structural formula for propane?
|
|
|
43.
|
A sample of gas occupies 17 mL at –112  C. What volume does
the sample occupy at 70  C? a. | 36mL | c. | 8.0mL | b. | 10.6 mL | d. | 27 mL |
|
|
|
44.
|
Aldehydes have the general structure ____________.
|
|
|
45.
|
The volume of a gas is reduced from 4 L to 0.5 L while the temperature is held
constant. How does the gas pressure change?
a. | It increases by a factor of two. | c. | It increases by a factor of
eight. | b. | It decreases by a factor of eight. | d. | It increases by a factor of
four. |
|
|
|
46.
|
How many carbons are in a molecule of hexane?
|
|
|
47.
|
When two substances react to form products, the reactant which is used up is
called the ____.
a. | excess reagent | c. | catalytic reagent | b. | limiting reagent | d. | determining
reagent |
|
|
|
48.
|
What instrument is normally used to measure atmospheric pressure?
a. | manometer | c. | vacuum | b. | thermometer | d. | barometer |
|
|
|
49.
|
What is the key difference between a liquid and a gas?
a. | average kinetic energy | c. | the motion of their particles | b. | intermolecular
attractions | d. | the ability to
flow |
|
|
|
50.
|
When one atom contributes both bonding electrons in a single covalent bond, the
bond is called a(n) ____.
a. | one-sided covalent bond | c. | ionic covalent
bond | b. | unequal covalent bond | d. | coordinate covalent bond |
|
|
|
51.
|
What type of hybridization occurs in the orbitals of a carbon atom participating
in a triple bond with another carbon atom?
|
|
|
52.
|
According to VSEPR theory, molecules adjust their shapes to keep which of the
following as far apart as possible?
a. | inner shell electrons | c. | mobile electrons | b. | pairs of valence electrons | d. | the electrons closest to the
nuclei |
|
|
|
53.
|
Chemical equations must be balanced to satisfy ____.
a. | Avogadro’s principle | c. | the law of conservation of
mass | b. | the law of definite proportions | d. | the law of multiple
proportions |
|
|
|
54.
|
Name the compound CH  CH  O CH  CH  CH  . a. | dipropyl ether | c. | ethylpropyl ether | b. | pentane oxide | d. | diethyl ether |
|
|
|
55.
|
Hydrocarbons containing a saturated carbon ring are called ____.
a. | alkylated hydrocarbons | c. | aliphatic hydrocarbons | b. | aromatic
hydrocarbons | d. | cyclic
hydrocarbons |
|
|
|
56.
|
What is the carbon skeleton of the product formed in the following reaction?
C  H  + HBr → a. |
 | c. |
 | b. |
CCBrC | d. |
CCCBr |
|
|
|
57.
|
Which of the following is a balanced equation representing the decomposition of
lead(IV) oxide?
|
|
|
58.
|
Chemical equations ____.
a. | describe chemical reactions | b. | describe only biological
changes | c. | give directions for naming chemical compounds | d. | show how to write
chemical formulas |
|
|
|
59.
|
Which of the following diatomic molecules is joined by a double covalent
bond?
|
|
|
60.
|
A catalyst is ____.
a. | a solid product of a reaction | b. | not used up in a reaction | c. | one of the reactants
in single-replacement reactions | d. | the product of a combustion
reaction |
|
|
|
61.
|
A bond formed between a silicon atom and an oxygen atom is likely to be
____.
a. | nonpolar covalent | c. | ionic | b. | polar covalent | d. | coordinate
covalent |
|
|
|
62.
|
When iron rusts in air, iron(III) oxide is produced. How many moles of oxygen
react with 2.4 mol of iron in the rusting reaction? 4Fe( s) + 3O  ( g) → 2Fe2O  ( s) a. | 2.4 mol | c. | 1.8 mol | b. | 3.2 mol | d. | 1.2 mol |
|
|
|
63.
|
What is the name of the functional group in the following compound?  a. | carbonyl | c. | carboxylic acid | b. | ester | d. | halogen |
|
|
|
64.
|
The longest continuous carbon chain of a branched-chain hydrocarbon is called
a(n) ____.
a. | substituted alkane | c. | principle alkane | b. | parent alkane | d. | isomer |
|
|
|
65.
|
Which of the following compounds is an unsaturated hydrocarbon?
a. | methyl | c. | nonane | b. | methane | d. | propyne |
|
|
|
66.
|
Which of the following atoms acquires the most negative charge in a covalent
bond with hydrogen?
|
|
|
67.
|
How many covalent bonds can each carbon atom form?
|
|
|
68.
|
Crystals are characterized by particular patterns that repeat in how many
dimensions?
a. | one dimension only | c. | The patterns do not repeat. | b. | three dimensions
only | d. | two dimensions
only |
|
|
|
69.
|
The calculation of quantities in chemical equations is called ____.
a. | stoichiometry | c. | percent composition | b. | percent yield | d. | dimensional
analysis |
|
|
|
70.
|
If a combination reaction takes place between rubidium and bromine, the chemical
formula for the product is ____.
a. | RbBr | c. | Rb Br Br | b. | RuBr | d. | RbBr |
|
|
|
71.
|
What are the coefficients that will balance the skeleton equation below?
N  + H   NH  a. | 3, 1, 2 | c. | 1, 3, 3 | b. | 1, 1, 2 | d. | 1, 3, 2 |
|
|
|
72.
|
In an addition reaction, which bond of the reactant is broken?
a. | carbon—hydrogen double bond | c. | carbon—carbon double
bond | b. | carbon—hydrogen single bond | d. | carbon—carbon single
bond |
|
Matching
|
|
|
Match each item with the correct statement below. a. | calorimeter | d. | enthalpy | b. | calorie | e. | specific heat | c. | joule | f. | heat
capacity |
|
|
|
73.
|
device used to measure the heat absorbed or released during a chemical or
physical process
|
|
|
74.
|
quantity of heat needed to raise the temperature of 1 g of water by 1 C C
|
|
|
75.
|
quantity of heat needed to change the temperature of 1 g of a substance by
1 C C
|
|
|
76.
|
heat content of a system at constant pressure
|
|
|
77.
|
SI unit of energy
|
|
|
78.
|
quantity of heat needed to change the temperature of an object by 1 C C
|