Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Which of the following statements are CORRECT? 1. For gas phase equilibria, the partial
pressures of reactants and products are equal.
2. For a chemical
system at equilibrium, the forward and reverse rates of reaction are
equal.
3. For an aqueous chemical system at equilibrium, the
concentrations of products divided by the concentrations of reactants equals one. a. | 1 only | b. | 2 only | c. | 3
only | d. | 1 and 2 | e. | 1, 2, and 3 |
|
|
|
2.
|
Which of the following statements are always CORRECT? 1. Product concentrations appear in the
numerator of an equilibrium constant expression.
2. A reaction
favors the formation of products if K >> 1.
3. Equilibrium
constants have units of atmospheres for gas phase reactions. a. | 1 only | b. | 2 only | c. | 3
only | d. | 1 and 2 | e. | 1, 2, and 3 |
|
|
|
3.
|
Write the expression for Kc for the reaction below.
Cu 2+(aq) + 4 NH 3(aq) 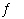
Cu(NH 3) 42+(aq)
|
|
|
4.
|
Write the expression for K for the reaction below.
Al 2S 3(s) 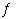 2 Al 3+(aq) + 3
S 2-(aq)
|
|
|
5.
|
Write the expression for K for the reaction of acetate ion with
water.
CH 3CO 2-(aq) + H 2O(?) 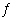
CH 3CO 2H(aq) + OH -(aq)
|
|
|
6.
|
Write a balanced chemical equation which corresponds to the following
equilibrium constant expression. a. | HF(aq) 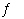 F-(aq) +
H3O+(aq) F-(aq) +
H3O+(aq) | b. | F-(aq) +
H3O+(aq) 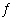 HF(aq) +
H2O(?) HF(aq) +
H2O(?) | c. | HF(aq) + H2O(?)
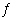 F-(aq) + H3O+(aq) F-(aq) + H3O+(aq) | d. | H+(aq) + OH-(aq) 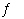 H2O(?)
H2O(?) | e. | F-(aq) +
H3O+(aq) 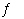 HF(aq) HF(aq) |
|
|
|
7.
|
What is the relationship between Kp and Kc
for the reaction below?
CS 2(g) + 3 Cl 2(g) 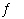 S 2Cl 2(g) + CCl 4(g)
|
|
|
8.
|
The oxidation of sulfur dioxide produces sulfur trioxide. 2
SO 2(g) + O 2(g) 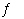 2
SO 3(g) Calculate the value of Kc, given that Kp =
2.8 × 10 2 at 999 K. ( R = 0.08206
L·atm/mol·K) a. | 4.4 × 10-5 | b. | 0.29 | c. | 3.4 | d. | 2.3 ×
104 | e. | 6.8 × 103 |
|
|
|
9.
|
A 4.00 L flask is filled with 0.75 mol SO 3, 2.50 mol SO 2,
and 1.30 mol O 2, and allowed to reach equilibrium. Predict the effect on the
concentrations of SO 3 as equilibrium is achieved by using Q, the reaction quotient.
Assume the temperature of the mixture is chosen so that Kc = 12. 2
SO 3(g) 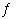 2 SO 2(g) +
O 2(g) a. | [SO3] will decrease because Q >
K. | b. | [SO3] will decrease because Q <
K. | c. | [SO3] will increase because Q <
K. | d. | [SO3] will increase because Q >
K. | e. | [SO3] will remain the same because Q =
K. |
|
|
|
10.
|
Consider the reaction A(g) 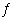 2 B(g) where Kp =
5.0 at 25 °C. If 0.50 mol A and 2.0 mol B are introduced into a 1.0 L flask at 25 °C, what
change in concentrations (if any) will occur in time? a. | [A] will decrease and [B] will decrease. | b. | [A] will
decrease and [B] will increase. | c. | [A] will increase and [B]
will increase. | d. | [A] will increase and [B] will decrease. | e. | [A] and
[B] remain unchanged. |
|
|
|
11.
|
At 25 °C, only 1.9 g CaSO 4 will
dissolve in 2.00 L of water. What is the equilibrium constant for the reaction below?
CaSO 4(s) 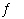 Ca 2+(aq) +
SO 42-(aq) a. | 4.9 × 10-5 | b. | 1.9 × 10-4 | c. | 1.4 ×
10-2 | d. | 7.0 × 10-3 | e. | 0.90 |
|
|
|
12.
|
When 0.20 mole NH 4Cl is dissolved in water to a volume of 1.00 L,
0.0053% of the NH 4+ dissociates to form NH 3. What is the equilibrium
constant for the reaction?
NH 4+(aq) + H 2O(?)
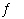 NH 3(aq) + H 3O +(aq) a. | 2.2 × 10-11 | b. | 1.1 × 10-10 | c. | 5.6 ×
10-10 | d. | 1.4 × 10-8 | e. | 5.6 × 10-6 |
|
|
|
13.
|
Nitrosyl bromide decomposes according to the chemical equation below. 2
NOBr(g) 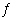 2 NO(g) + Br 2(g) When
0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium, 22% of the NOBr decomposes.
What is the equilibrium constant, Kp, for the reaction? a. | 2.3 × 10-3 | b. | 4.5 × 10-3 | c. | 3.5 ×
10-2 | d. | 4.8 × 10-2 | e. | 8.0 × 10-2 |
|
|
|
14.
|
The equilibrium constant at 25 °C for the dissolution of silver bromide is
5.4 × 10 -13. AgBr(s)
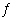 Ag +(aq) + Br -(aq) If an excess quantity of
AgBr(s) is added to water and allowed to equilibrate, what is the equilibrium concentration of
Ag +? a. | 2.9 × 10-25 M | b. | 2.7 × 10-13 M | c. | 5.4 ×
10-13 M | d. | 1.1 ×
10-12 M | e. | 7.3 ×
10-7 M |
|
|
|
15.
|
Given the following equilibria, Ni 2+(aq) + 2
OH -(aq) 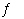
Ni(OH) 2(s)
K1 = 1.8 × 10 15
Ni(NH 3) 62+(aq) 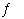 Ni 2+(aq)
+ 6 NH 3(aq)
K2 = 5.6 ×
10 8determine the equilibrium constant for the following
reaction. Ni(OH) 2(s) + 6
NH 3(aq) 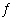
Ni(NH 3) 62+(aq) + 2
OH -(aq) K3a. | 9.9 × 10-25 | c. | 3.1 × 10-7 | b. | 1.8 ×
10-9 | d. | 3.2 × 106 e)1.0 × 1024 |
|
|
|
16.
|
Assume that the following chemical reaction is at equilibrium.
I 2(g) + Cl 2(g) 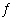 2
ICl(g) ÄH° 2
ICl(g) ÄH°
= -26.9 kJ At 25 °C, Kp = 2.0 × 10 5. If the temperature is decreased to 5 °C, which statement applies? a. | Kp will decrease and the reaction will proceed in the forward
direction. | b. | Kp will decrease and the reaction will proceed in the backward
direction. | c. | Kp will remain unchanged and the reaction will proceed in the
forward direction. | d. | Kp will remain unchanged and
the reaction will proceed in the backward direction. | e. | Kp will increase and the
reaction will proceed in the forward direction. |
|
|
|
17.
|
Assume that the following endothermic chemical reaction is at
equilibrium.
C(s) + H 2O(g) 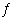
H 2(g) + CO(g) Which of the following statements are CORRECT? 1. Increasing the amount of C(s) will increase
the equilibrium concentration of CO(g).
2. Increasing the
temperature will increase the equilibrium concentration of
H2(g).
3. Decreasing the concentration of
H2O(g) will increase the equilibrium concentration of CO(g). a. | 1 only | b. | 2 only | c. | 3
only | d. | 1 and 2 | e. | 1, 2, and 3 |
|
|
|
18.
|
In which of the following equilibrium systems will an increase in the pressure
have NO effect on the concentrations of products and reactants?
a. | CaO(s) + CO2(g) 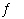 CaCO3(s)
CaCO3(s) | b. | N2(g) + 3
H2(g) 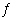 2 NH3(g) 2 NH3(g) | c. | H2(g) + Cl2(g) 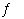 2
HCl(g) 2
HCl(g) | d. | H2(g) + CO2(g) 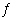 CO(g)
+ H2O(?) CO(g)
+ H2O(?) | e. | 2 H2O2(g) 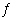 2 H2O(g) + O2(g) 2 H2O(g) + O2(g) |
|