Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
What is the temperature –34 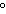 C expressed in kelvins? a. | 139 K | c. | 239 K | b. | 207 K | d. | 339 K |
|
|
|
2.
|
The letter " p" in the symbol 4 p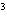
indicates the ____. a. | spin of an electron | c. | principle energy level | b. | orbital
shape | d. | speed of an
electron |
|
|
|
3.
|
The chemical symbol for iron is ____.
|
|
|
4.
|
What is the density of an object having a mass of 8.0 g and a volume of 25
cm 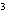 ? a. | 0.32 g/cm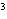 | c. | 3.1 g/cm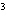 | b. | 2.0 g/cm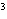 | d. | 200
g/cm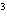 |
|
|
|
5.
|
How does calcium obey the octet rule when reacting to form compounds?
a. | It gains electrons. | b. | It gives up electrons. | c. | It does not change
its number of electrons. | d. | Calcium does not obey the octet
rule. |
|
|
|
6.
|
Which of the following is a heterogeneous mixture?
a. | vinegar in water | c. | oil and vinegar | b. | milk | d. | air |
|
|
|
7.
|
Which of the following shows correctly an ion pair and the ionic compound the
two ions form?
|
|
|
8.
|
Which of the following can be observed only in a microscopic view?
a. | foam insulation | c. | shape of a soybean plant | b. | X-ray of a knee
joint | d. | structure of a muscle
cell |
|
|
|
9.
|
The quantum mechanical model of the atom ____.
a. | defines the exact path of an electron around the nucleus | b. | was proposed by
Niels Bohr | c. | involves the probability of finding an electron in a certain
position | d. | has many analogies in the visible world |
|
|
|
10.
|
The closeness of a measurement to its true value is a measure of its
____.
a. | precision | c. | reproducibility | b. | accuracy | d. | usefulness |
|
|
|
11.
|
What is the wavelength of an electromagnetic wave that travels at 3 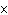 10 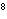 m/s and has a frequency of 60 MHz? (1 MHz =
1,000,000 Hz) a. | 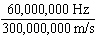 | b. | 60 MHz ×
300,000,000 m/s | c. | 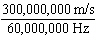 | d. | No answer can be determined from the
information given. |
|
|
|
12.
|
What is the correct name for Sn 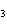 (PO 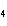 ) 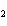 ? a. | tritin diphosphate | c. | tin(III) phosphate | b. | tin(II) phosphate | d. | tin(IV)
phosphate |
|
|
|
13.
|
What is the volume of 45.6 g of silver if the density of silver is 10.5
g/mL?
a. | 0.23 mL | c. | 479 mL | b. | 4.34 mL | d. | none of the
above |
|
|
|
14.
|
Which state of matter takes both the shape and volume of its container?
a. | solid | c. | gas | b. | liquid | d. | both b and c |
|
|
|
15.
|
In which of the following sets are the charges given correctly for all the
ions?
|
|
|
16.
|
What is one difference between a mixture and a compound?
a. | A compound consists of more than one phase. | b. | A compound can only
be separated into its components by chemical means. | c. | A mixture can only be separated into its
components by chemical means. | d. | A mixture must be uniform in
composition. |
|
|
|
17.
|
Which of the following compounds contains the lead(II) ion?
|
|
|
18.
|
What is the shape of the 3p atomic orbital?
a. | sphere | c. | bar | b. | dumbbell | d. | two perpendicular
dumbbells |
|
|
|
19.
|
What is another name for the transition metals?
a. | noble gases | c. | Group B elements | b. | Group A elements | d. | Group C
elements |
|
|
|
20.
|
How can the position of a particle be determined?
a. | by analyzing its interactions with another particle | b. | by measuring its
velocity | c. | by measuring its mass | d. | by determining its
charge |
|
|
|
21.
|
The atomic mass of an element is the ____.
a. | total number of subatomic particles in its nucleus | b. | weighted average of
the masses of the isotopes of the element | c. | total mass of the isotopes of the
element | d. | average of the mass number and the atomic number for the
element |
|
|
|
22.
|
What does the number 84 in the name krypton-84 represent?
a. | the atomic number | c. | the sum of the protons and electrons | b. | the mass
number | d. | twice the number of
protons |
|
|
|
23.
|
What is the correct formula for barium chlorate?
a. | Ba(ClO)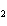 | c. | Ba(ClO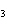 ) )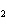 | b. | Ba(ClO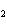 ) )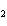 | d. | BaCl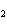 |
|
|
|
24.
|
The atomic number of an element is the total number of which particles in the
nucleus?
a. | neutrons | c. | electrons | b. | protons | d. | protons and
electrons |
|
|
|
25.
|
What is the charge on the cation in the ionic compound sodium sulfide?
|
|
|
26.
|
Which of the following elements has the smallest first ionization energy?
a. | sodium | c. | potassium | b. | calcium | d. | magnesium |
|
|
|
27.
|
What is the charge of a cation?
a. | a positive charge | b. | no charge | c. | a negative
charge | d. | The charge depends on the size of the nucleus. |
|
|
|
28.
|
What is the electron configuration of the calcium ion?
|
|
|
29.
|
The diameter of a carbon atom is 0.000 000 000 154 m. What is this number
expressed in scientific notation?
|
|
|
30.
|
A vapor is which state of matter?
a. | solid | c. | gas | b. | liquid | d. | all of the
above |
|
|
|
31.
|
What is the element with the highest electronegativity value?
a. | cesium | c. | calcium | b. | helium | d. | fluorine |
|
|
|
32.
|
A chemical change occurs when a piece of wood ____.
a. | is split | c. | decays | b. | is painted | d. | is cut |
|
|
|
33.
|
What is the formula unit of sodium nitride?
|
|
|
34.
|
Which of the following is a chemical property?
a. | color | c. | freezing point | b. | hardness | d. | ability to react with
oxygen |
|
|
|
35.
|
When dissolved in water, acids produce ____.
a. | negative ions | c. | hydrogen ions | b. | polyatomic ions | d. | oxide ions |
|
|
|
36.
|
Which of the following electromagnetic waves have the highest
frequencies?
a. | ultraviolet light waves | c. | microwaves | b. | X-rays | d. | gamma rays |
|
|
|
37.
|
How do the isotopes hydrogen-1 and hydrogen-2 differ?
a. | Hydrogen-2 has one more electron than hydrogen-1. | b. | Hydrogen-2 has one
neutron; hydrogen-1 has none. | c. | Hydrogen-2 has two protons; hydrogen-1 has
one. | d. | Hydrogen-2 has one proton; hydrogen-1 has none. |
|
|
|
38.
|
What is the next atomic orbital in the series 1s, 2s, 2p,
3s, 3p?
|
|
|
39.
|
Which of the following is NOT an example of matter?
a. | air | c. | smoke | b. | heat | d. | water vapor |
|
|
|
40.
|
Which of the following was originally a tenet of Dalton's atomic theory,
but had to be revised about a century ago?
a. | Atoms are tiny indivisible particles. | b. | Atoms of the same element are
identical. | c. | Compounds are made by combining atoms. | d. | Atoms of different elements can combine with
one another in simple whole number ratios. |
|
|
|
41.
|
How many electrons does nitrogen gain in order to achieve a noble-gas electron
configuration?
|
|
|
42.
|
An -ate or -ite at the end of a compound name usually indicates
that the compound contains ____.
a. | fewer electrons than protons | c. | only two
elements | b. | neutral molecules | d. | a polyatomic anion |
|
|
|
43.
|
What is the Stock name for chromic ion?
a. | chromium(I) ion | c. | chromium(III) ion | b. | chromium(II) ion | d. | chromium(IV)
ion |
|
|
|
44.
|
What is the maximum number of f orbitals in any single energy level in an
atom?
|
|
|
45.
|
Which of the following quantum leaps would be associated with the greatest
energy of emitted light?
a. | n = 5 to n = 1 | c. | n = 1 to n = 7 | b. | n = 4 to n = 5 | d. | n = 5 to n = 4 |
|
|
|
46.
|
Which of the following groupings contains only representative elements?
a. | Cu, Co, Cd | c. | Al, Mg, Li | b. | Ni, Fe, Zn | d. | Hg, Cr, Ag |
|
|
|
47.
|
How does oxygen obey the octet rule when reacting to form compounds?
a. | It gains electrons. | b. | It gives up electrons. | c. | It does not change
its number of electrons. | d. | Oxygen does not obey the octet
rule. |
|
|
|
48.
|
All atoms are ____.
a. | positively charged, with the number of protons exceeding the number of
electrons | b. | negatively charged, with the number of electrons exceeding the number of
protons | c. | neutral, with the number of protons equaling the number of
electrons | d. | neutral, with the number of protons equaling the number of electrons, which is equal
to the number of neutrons |
|
|
|
|
|
|
49.
|
What is the quantity 7896 millimeters expressed in meters? Use the table above
to help you.
a. | 7.896 m | c. | 789.6 m | b. | 78.96 m | d. | 789,600 m |
|
|
|
50.
|
What is the quantity 0.0075 meters expressed in centimeters? Use the table above
to help you.
a. | 0.075 cm | c. | 7.5 cm | b. | 0.75 cm | d. | 70.5 cm |
|
|
|
51.
|
Which of the following measurements contains two significant figures?
a. | 0.004 00 L | c. | 0.000 44 L | b. | 0.004 04 L | d. | 0.004 40 L |
|
|
|
52.
|
How does atomic radius change from left to right across a period in the periodic
table?
a. | It tends to decrease. | c. | It first increases, then decreases. | b. | It tends to
increase. | d. | It first
decreases, then increases. |
|
|
|
53.
|
Which of the following volumes is the smallest?
a. | one microliter | c. | one milliliter | b. | one liter | d. | one deciliter |
|
|
|
54.
|
Atomic size generally ____.
a. | increases as you move from left to right across a period | b. | decreases as you
move from top to bottom within a group | c. | remains constant within a
period | d. | decreases as you move from left to right across a
period |
|
|
|
55.
|
What is the approximate energy of a photon having a frequency of 4 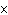 10 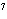 Hz? ( h = 6.6 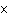 10 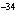 J  s)
|
|
|
56.
|
What is the correct formula for potassium sulfite?
|
|
|
57.
|
Express the product of 2.2 mm and 5.00 mm using the correct number of
significant digits.
a. | 10 mm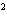 | c. | 11.0 mm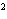 | b. | 11 mm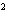 | d. | 11.00
mm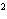 |
|
|
|
58.
|
Ionic compounds are normally in which physical state at room temperature?
a. | solid | c. | gas | b. | liquid | d. | plasma |
|
|
|
59.
|
How many valence electrons are in an atom of magnesium?
|
|
|
60.
|
Which step in the scientific method requires you to use your senses to obtain
information?
a. | revising a hypothesis | c. | making an observation | b. | designing an
experiment | d. | stating a
theory |
|
|
|
61.
|
In which of the following sets is the symbol of the element, the number of
protons, and the number of electrons given correctly?
a. | In, 49 protons, 49 electrons | c. | Cs, 55 protons, 132.9
electrons | b. | Zn, 30 protons, 60 electrons | d. | F, 19 protons, 19
electrons |
|
|
|
62.
|
Aluminum is a group 3A metal. Which ion does A1 typically form?
|
|
|
63.
|
Which of the following best describes an example of pure chemistry?
a. | testing the effects of lower concentrations of a drug on humans | b. | studying chemicals
containing carbon | c. | developing a cure for
osteoporosis | d. | finding an antidote for a new strain of virus |
|
|
|
64.
|
Which of the following is a physical change?
a. | corrosion | c. | evaporation | b. | explosion | d. | rotting of food |
|
|
|
65.
|
An example of an extensive property of matter is ____.
a. | temperature | c. | mass | b. | pressure | d. | hardness |
|
|
|
66.
|
Which of the following particles are free to drift in metals?
a. | protons | c. | neutrons | b. | electrons | d. | cations |
|
|
|
67.
|
Which group of measurements is the most precise? (Each group of measurements is
for a different object.)
a. | 2 g, 3 g, 4 g | c. | 2 g, 2.5 g, 3 g | b. | 2.0 g, 3.0 g, 4.0 g | d. | 1 g, 3 g, 5 g |
|
|
|
68.
|
What happens to matter during a chemical reaction?
a. | Matter is neither destroyed or created. | b. | Some matter is
destroyed. | c. | Some matter is created. | d. | Some matter is destroyed and some is
created. |
|
|
|
69.
|
What is the formula for sodium sulfate?
|
|
|
70.
|
The modern periodic table is arranged in order of increasing atomic ____.
a. | mass | c. | number | b. | charge | d. | radius |
|
|
|
71.
|
Which of the following is NOT a physical property of water?
a. | It has a boiling point of 100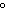 C. C. | b. | It is a colorless
liquid. | c. | It is composed of hydrogen and oxygen. | d. | Sugar dissolves in
it. |
|
|
|
72.
|
Which color of visible light has the shortest wavelength?
a. | yellow | c. | blue | b. | green | d. | violet |
|
|
|
73.
|
When an iron nail is ground into powder, its mass ____.
a. | stays the same | c. | increases | b. | decreases | d. | cannot be
determined |
|
|
|
74.
|
What is the formula for phosphoric acid?
|
|
|
75.
|
Which of the following is true about subatomic particles?
a. | Electrons are negatively charged and are the heaviest subatomic
particle. | b. | Protons are positively charged and the lightest subatomic
particle. | c. | Neutrons have no charge and are the lightest subatomic particle. | d. | The mass of a
neutron nearly equals the mass of a proton. |
|
|
|
76.
|
What SI unit is used to measure the number of representative particles in a
substance?
a. | kilogram | c. | kelvin | b. | ampere | d. | mole |
|
|
|
77.
|
All of the following are equal to Avogadro's number EXCEPT ____.
a. | the number of atoms of bromine in 1 mol Br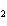 | b. | the number of atoms of gold in 1 mol
Au | c. | the number of molecules of nitrogen in 1 mol N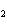 | d. | the number of molecules of carbon monoxide in 1
mol CO |
|
|
|
78.
|
How many moles of silver atoms are in 1.8 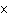 10 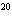 atoms of silver?
|
|
|
79.
|
How many atoms are in 0.075 mol of titanium?
|
|
|
80.
|
The atomic masses of any two elements contain the same number of ____.
a. | atoms | c. | ions | b. | grams | d. | milliliters |
|
|
|
81.
|
What is the molar mass of AuCl3?
a. | 96 g | c. | 232.5 g | b. | 130 g | d. | 303.6 g |
|
|
|
82.
|
What is the number of moles of beryllium atoms in 36 g of Be?
a. | 0.25 mol | c. | 45.0 mol | b. | 4.0 mol | d. | 320 mol |
|
|
|
83.
|
The molar volume of a gas at STP occupies ____.
a. | 22.4 L | c. | 1 kilopascal | b. | 0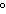 C C | d. | 12
grams |
|
|
|
84.
|
What is the percent composition of chromium in BaCrO 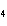 ? a. | 4.87% | c. | 20.5% | b. | 9.47% | d. | 25.2% |
|
|
|
85.
|
What is the percent by mass of carbon in acetone, C 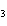 H 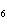 O? a. | 20.7% | c. | 1.61% | b. | 62.1% | d. | 30.0% |
|
|
|
86.
|
What is the empirical formula of a substance that is 53.5% C, 15.5% H, and 31.1%
N by weight?
|
|
|
87.
|
The ratio of carbon atoms to hydrogen atoms to oxygen atoms in a molecule of
dicyclohexyl maleate is 4 to 6 to 1. What is its molecular formula if its molar mass is 280 g?
|
Short Answer
|
|
|
88.
|
From which orbital in a lithium atom is an electron transferred to form Li 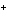 ?
|
|
|
89.
|
What is the sum of 6.210 L and 3 L expressed in the correct number of
significant digits?
|
|
|
90.
|
What is the total number of subatomic particles in the nucleus of an atom of
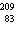 Bi?
|
|
|
91.
|
Chlorine has two naturally occurring isotopes, Cl-35 and Cl-37. The atomic mass
of chlorine is 35.45. Which of these two isotopes of chlorine is more abundant?
|
|
|
92.
|
How many grams of liquid water are produced when 60 grams of ice melt?
|
|
|
93.
|
How many protons are present in an atom of Be-9?
|
|
|
94.
|
How many electrons does the ion Ca 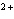 contain?
|
|
|
95.
|
How many electrons are in the highest occupied energy level of a neutral
chlorine atom?
|
|
|
96.
|
What is the charge on the cation in CuSO 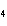 ?
|
|
|
97.
|
Write the formula for the compound rubidium phosphide.
|
|
|
98.
|
Find the mass in grams of 3.10 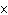 10 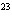 molecules of F 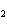 .
|
|
|
99.
|
Find the mass, in grams, of 1.40 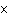 10 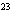 molecules of N 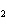 .
|
|
|
100.
|
What is the percent composition of NiO, if a sample of NiO with a mass of 41.9 g
contains 33.1 g Ni and 8.8 g O?
|