Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Which set of chemical name and chemical formula for the same compound is
correct?
a. | ammonium sulfite, (NH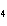 ) )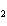 S S | c. | lithium carbonate, LiCO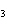 | b. | iron(III) phosphate,
FePO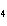 | d. | magnesium dichromate, MgCrO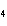 |
|
|
|
2.
|
In which of the following are the formula of the ionic compound and the charge
on the metal ion shown correctly?
|
|
|
3.
|
How many electrons does nitrogen gain in order to achieve a noble-gas electron
configuration?
|
|
|
4.
|
Aluminum is a group 3A metal. Which ion does A1 typically form?
|
|
|
5.
|
What determines that an element is a metal?
a. | the magnitude of its charge | c. | when it is a Group A
element | b. | the molecules that it forms | d. | its position in the periodic table |
|
|
|
6.
|
Which of the following compounds has the formula KNO 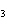 ? a. | potassium nitrate | c. | potassium nitrite | b. | potassium nitride | d. | potassium nitrogen
oxide |
|
|
|
7.
|
How does oxygen obey the octet rule when reacting to form compounds?
a. | It gains electrons. | b. | It gives up electrons. | c. | It does not change
its number of electrons. | d. | Oxygen does not obey the octet
rule. |
|
|
|
8.
|
Which polyatomic ion forms a neutral compound when combined with a group 1A
monatomic ion in a 1:1 ratio?
a. | ammonium | c. | nitrate | b. | carbonate | d. | phosphate |
|
|
|
9.
|
What is the correct formula for barium chlorate?
a. | Ba(ClO)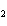 | c. | Ba(ClO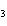 ) )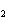 | b. | Ba(ClO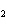 ) )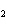 | d. | BaCl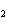 |
|
|
|
10.
|
Which of the following shows both the correct formula and correct name of an
acid?
a. | HClO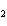 , chloric acid , chloric acid | c. | H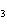 PO PO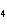 , phosphoric acid , phosphoric acid | b. | HNO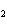 , hydronitrous acid , hydronitrous acid | d. | HI, iodic acid |
|
|
|
11.
|
What is the formula unit of sodium nitride?
|
|
|
12.
|
Which of the following correctly provides the names and formulas of polyatomic
ions?
|
|
|
13.
|
What is the electron configuration of the calcium ion?
|
|
|
14.
|
Which of the following are produced when a base is dissolved in water?
a. | hydronium ions | c. | hydrogen ions | b. | hydroxide ions | d. | ammonium ions |
|
|
|
15.
|
What is the net charge of the ionic compound calcium fluoride?
a. | 2– | c. | 0 | b. | 1– | d. | 1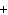 |
|
|
|
16.
|
Select the correct formula for sulfur hexafluoride.
|
|
|
17.
|
What is the Stock name for chromic ion?
a. | chromium(I) ion | c. | chromium(III) ion | b. | chromium(II) ion | d. | chromium(IV)
ion |
|
|
|
18.
|
How many valence electrons are in an atom of magnesium?
|
|
|
19.
|
Which of the following compounds contains the lead(II) ion?
|
|
|
20.
|
Which set of chemical name and chemical formula for the same compound is
correct?
a. | iron(II) oxide, Fe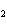 O O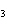 | c. | tin(IV) bromide, SnBr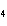 | b. | aluminum fluorate,
AlF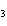 | d. | potassium chloride, K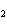 Cl Cl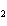 |
|
|
|
21.
|
Ionic compounds are normally in which physical state at room temperature?
a. | solid | c. | gas | b. | liquid | d. | plasma |
|
|
|
22.
|
What is the correct formula for potassium sulfite?
|
|
|
23.
|
Which of the following particles are free to drift in metals?
a. | protons | c. | neutrons | b. | electrons | d. | cations |
|
|
|
24.
|
What is the correct name for Sn 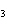 (PO 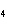 ) 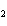 ? a. | tritin diphosphate | c. | tin(III) phosphate | b. | tin(II) phosphate | d. | tin(IV)
phosphate |
|
|
|
25.
|
Which element, when combined with fluorine, would most likely form an ionic
compound?
a. | lithium | c. | phosphorus | b. | carbon | d. | chlorine |
|
|
|
26.
|
What is the electron configuration of the oxide ion (O 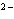 )?
|
|
|
27.
|
What does an -ite or -ate ending in a polyatomic ion mean?
a. | Oxygen is in the formula. | c. | Nitrogen is in the
formula. | b. | Sulfur is in the formula. | d. | Bromine is in the formula. |
|
|
|
28.
|
What is the formula for sodium sulfate?
|
|
|
29.
|
How many valence electrons are in a silicon atom?
|
|
|
30.
|
What is the formula for sulfurous acid?
|
|
|
31.
|
How does calcium obey the octet rule when reacting to form compounds?
a. | It gains electrons. | b. | It gives up electrons. | c. | It does not change
its number of electrons. | d. | Calcium does not obey the octet
rule. |
|
|
|
32.
|
Which of the following compounds contains the Mn 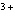 ion?
|
|
|
33.
|
Which of the following is NOT a cation?
a. | iron(III) ion | c. | Ca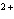 | b. | sulfate | d. | mercurous ion |
|
|
|
34.
|
An -ate or -ite at the end of a compound name usually indicates
that the compound contains ____.
a. | fewer electrons than protons | c. | only two
elements | b. | neutral molecules | d. | a polyatomic anion |
|
|
|
35.
|
Which of the following shows correctly an ion pair and the ionic compound the
two ions form?
|
|
|
36.
|
What is the formula for phosphoric acid?
|
|
|
37.
|
What is the charge on the cation in the ionic compound sodium sulfide?
|
|
|
38.
|
When dissolved in water, acids produce ____.
a. | negative ions | c. | hydrogen ions | b. | polyatomic ions | d. | oxide ions |
|
|
|
39.
|
What is the correct name for the compound CoCl 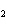 ? a. | cobalt(I) chlorate | c. | cobalt(II) chlorate | b. | cobalt(I) chloride | d. | cobalt(II)
chloride |
|
|
|
40.
|
What is the name of the ionic compound formed from lithium and bromine?
a. | lithium bromine | c. | lithium bromium | b. | lithium bromide | d. | lithium bromate |
|
Short Answer
|
|
|
41.
|
How many electrons does a gallium atom give up when it becomes an ion?
|
|
|
42.
|
Give the electron configuration for calcium the ion.
|
|
|
43.
|
Give the electron configuration for the lithium ion.
|
|
|
44.
|
How many valence electrons are in bromine?
|
|
|
45.
|
Write the formula for the compound rubidium phosphide.
|
|
|
46.
|
Write the formula for the compound barium oxide.
|
|
|
47.
|
What is the charge on the cation in CuSO 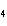 ?
|
Numeric Response
|
|
|
48.
|
Determine the subscript for ammonium in the chemical formula for ammonium
dichromate.
|
|
|
49.
|
What is the charge on the polyatomic ions nitrite and chlorite?
|
|
|
50.
|
What is the charge of a particle having 9 protons and 10 electrons?
|