Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Which of the following was originally a tenet of Dalton's atomic theory,
but had to be revised about a century ago?
a. | Atoms are tiny indivisible particles. | b. | Atoms of the same element are
identical. | c. | Compounds are made by combining atoms. | d. | Atoms of different elements can combine with
one another in simple whole number ratios. |
|
|
|
2.
|
Why did J. J. Thomson reason that electrons must be a part of the atoms of all
elements?
a. | Cathode rays are negatively-charged particles. | b. | Cathode rays can be
deflected by magnets. | c. | An electron is 2000 times lighter than a
hydrogen atom. | d. | Charge-to-mass ratio of electrons was the same, regardless of the gas
used. |
|
|
|
3.
|
Which of the following is true about subatomic particles?
a. | Electrons are negatively charged and are the heaviest subatomic
particle. | b. | Protons are positively charged and the lightest subatomic
particle. | c. | Neutrons have no charge and are the lightest subatomic particle. | d. | The mass of a
neutron nearly equals the mass of a proton. |
|
|
|
4.
|
Who conducted experiments to determine the quantity of charge carried by an
electron?
a. | Rutherford | c. | Dalton | b. | Millikan | d. | Thomson |
|
|
|
5.
|
What is the relative mass of an electron?
a. | 1/1840 the mass of a hydrogen atom | c. | 1/1840 the mass of a C-12
atom | b. | 1/1840 the mass of a neutron + proton | d. | 1/1840 the mass of an alpha
particle |
|
|
|
6.
|
Which hypothesis led to the discovery of the proton?
a. | When a neutral hydrogen atom loses an electron, a positively-charged particle should
remain. | b. | A proton should be 1840 times heavier than an electron. | c. | Cathode rays should
be attracted to a positively-charged plate. | d. | The nucleus of an atom should contain
neutrons. |
|
|
|
7.
|
What does the number 84 in the name krypton-84 represent?
a. | the atomic number | c. | the sum of the protons and electrons | b. | the mass
number | d. | twice the number of
protons |
|
|
|
8.
|
In which of the following sets is the symbol of the element, the number of
protons, and the number of electrons given correctly?
a. | In, 49 protons, 49 electrons | c. | Cs, 55 protons, 132.9
electrons | b. | Zn, 30 protons, 60 electrons | d. | F, 19 protons, 19
electrons |
|
|
|
9.
|
Using the periodic table, determine the number of neutrons in 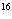 O.
|
|
|
10.
|
How many protons, electrons, and neutrons does an atom with atomic number 50 and
mass number 125 contain?
a. | 50 protons, 50 electrons, 75 neutrons | c. | 120 neutrons, 50 protons, 75
electrons | b. | 75 electrons, 50 protons, 50 neutrons | d. | 70 neutrons, 75 protons, 50
electrons |
|
|
|
11.
|
In which of the following is the number of neutrons correctly
represented?
a. | 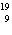 F has 0 neutrons. F has 0 neutrons. | c. | 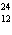 Mg has 24
neutrons. Mg has 24
neutrons. | b. | 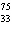 As has 108 neutrons. As has 108 neutrons. | d. | 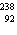 U has 146
neutrons. U has 146
neutrons. |
|
|
|
12.
|
Which of the following isotopes has the same number of neutrons as
phosphorus-31?
|
|
|
13.
|
What unit is used to measure weighted average atomic mass?
a. | amu | c. | angstrom | b. | gram | d. | nanogram |
|
|
|
14.
|
Which of the following equals one atomic mass unit?
a. | the mass of one electron | b. | the mass of one helium-4
atom | c. | the mass of one carbon-12 atom | d. | one-twelfth the mass of one carbon-12
atom |
|
|
|
15.
|
Which of the following statements is NOT true?
a. | Protons have a positive charge. | b. | Electrons are negatively charged and have a
mass of 1 amu. | c. | The nucleus of an atom is positively charged. | d. | Neutrons are located
in the nucleus of an atom. |
|
Short Answer
|
|
|
16.
|
A fictitious element X is composed of 10.0 percent of the isotope 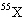 , 20.0 percent of the isotope 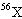 , and 70.0 percent of the
isotope 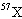 . Estimate the atomic mass of element X.
|
Numeric Response
|
|
|
17.
|
What is the relative charge of a proton?
|
|
|
18.
|
Determine the number of electrons in an atom of iridium.
|
|
|
19.
|
What is the atomic number for an element with 41 neutrons and a mass number of
80?
|
Essay
|
|
|
20.
|
What observations by Rutherford led to the hypothesis that atoms are mostly
empty space, and that almost all of the mass of the atom is contained in an atomic nucleus?
|